Here’s a hot flash you may not have heard before. You’re not aging. You’re rusting. As we get older, we start to face a complexion that looks duller, dingy, cloudier, less luminous. And more unevenly discolored. The reason? Iron is the most abundant transition metal in human body and the best-known driving force behind oxygen free radical formation through Fenton and Haber-Weiss reactions (Pierre and Fontecave 1999). Yes, the irony of iron is that iron is the element essential for building strong structures and machines but can also be the cause of their deterioration through rust. Oxidative damage a.k.a. – rusting is due to two distinct but surprisingly related functions: the build-up of iron in the skin as we get older coupled with a slowing of the skin’s natural exfoliation process. These result in iron staying in the skin for 56 days instead of 28 days as skin turnover time (Weintraub et al. 1965).
Here’s another conundrum. In pre-menopause, excess iron in the body and skin is eliminated two ways: through monthly menstruation and through exfoliation. When menstruation ceases, iron levels can surge. Excess iron in the body is common with the onset of menopause. In fact, iron can accumulate in the skin and is clinically shown to increase by as much as +42% pre through post menopause (Pelle et al. 2013). This excess iron is normally eliminated through menstruation as well as natural exfoliation. The cessation of menstruation coupled with the deceleration of exfoliation limits the capacity to remove excess iron from the skin.
When body iron storage increases, skin too is exposed to higher levels of iron, which in turn can cause oxidative damage as the excess iron reacts with UVA. The result: skin aging and photoaging is accelerated. For example, ferritin is the iron storage protein for excess iron with a capacity of binding up to 4,500 atoms of iron per ferritin. It consists of heavy (H) and light (L) chain subunits. It was shown that ferritin can be immediately degraded by UVA doses of 100 and 250 kJ/m2, releasing large amounts of iron for Fenton and Haber-Weiss reactions, producing oxygen free radicals (Pourzand et al. 1999). In a cell culture model mimicking menopausal conditions, increased iron and UVA were found to significantly increase an enzyme called collagenase-1 (Jian et al. 2011). Increased activity of collagenase-1 increases collagen degradation, causing wrinkles initially and skin thinning when we get older.
Like rust on metal, higher levels of iron when exposed to UV radiation, blue light, air pollutants and irritants from other sources results in increased oxidative damage in skin. If you’ve ever seen a rusty bicycle, you know the corrosive damage the environment has on metal. Oxidative stress begets skin-aging yellowing, dullness, dark spots and discolorations as well as wrinkles, loss of elasticity and other signs of aging.
Until now, antioxidants have been the only defense to attempt to neutralize oxidants after they are formed. Chelation can be used to sequester “free” iron, but it cannot compete to take away from iron in ferritin. The antioxidant and chelation approaches are retroactive and often too late (Table 1). They can only battle the symptoms, but they do not treat the underlying cause.
Table 1. Mode of actions of antioxidants, chelation, and de-ironizing inducer (DII) *
| Antioxidants | Chelation | De-ironizing inducer (DII) |
| Reduce damage
Attempt to reduce the cumulative damage to skin by neutralizing some of the radicals before they can damage skin |
Diminish damage
Try to sequester “free” iron is not bound to proteins. Chelation is reversible, and chelators cannot compete with ferritin, the strongest iron chelator |
Prevent damage
DII consists of ascorbic acids and pearl powder to safely remove iron from ferritin, the landmine forming oxygen free radicals |
| Retro-active
Antioxidants fight oxidants |
Reversible and incomplete Ineffective protection | Proactive
DII stops skin aging one step earlier than antioxidants and more complete than chelation. |
*: The skin is subject to a constant onslaught of free radicals catalyzed by iron-mediated Fenton and Haber-Weiss reactions.
A novel class of actives termed De-ironizing Inducers Technology (DII®) can do what no free-radical neutralizing antioxidant or chelation can do. It reduces iron in ferritin before it is converted to skin-damaging free radicals. The patented DII® features 3-o-ethyl-ascorbic acid and pearl powder in the right ratios to effectively reduce iron in skin (US Patent 10792240). Research shows that when either alone or when too much of one exists without the other, iron reduction cannot be accomplished. Ascorbic acid, also known as vitamin C helps release iron deposits from ferritin. Pearl Powder, a soft form of calcium carbonate, absorbs the released iron ions by exchanging them with calcium ions and, thus, removes skin-rusting iron deposits (Figure 1). These two previously incompatible ingredients work in unison to help prevent oxidative stress from forming in the first place, rather than attempting to neutralize free radicals after they appear as most antioxidants do. Without the need to fight free radicals, skin-rejuvenating Hyaluronic Acid plus Tetrapeptide-11 repair previous damage done and the goes one step further to help rebuild collagen, clarity, elasticity and tone / skin’s health and appearance.
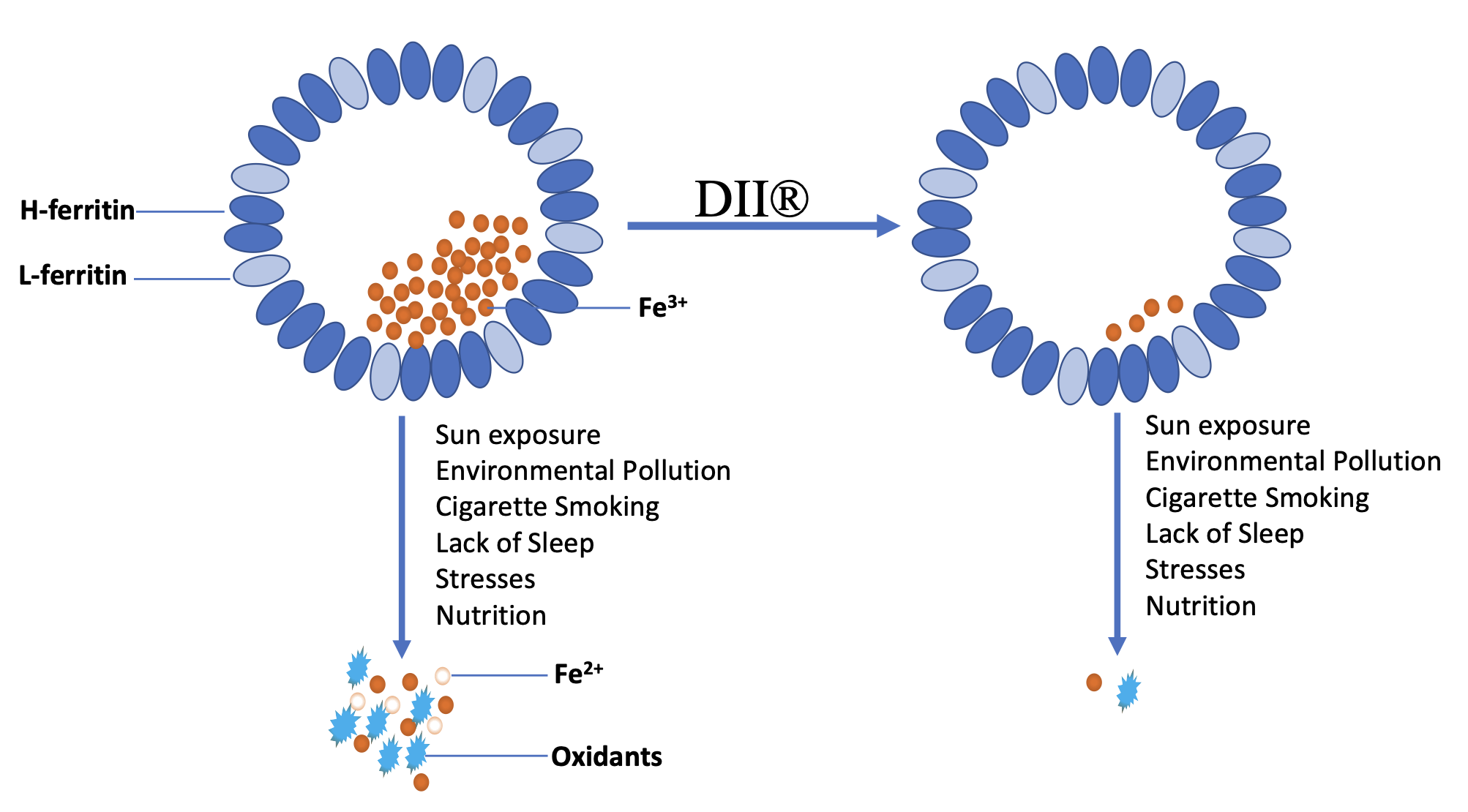
Your skin glows with good health. And rust is history!
It is important to note that, while this discovery is made using menopausal model, the DII® is applicable to all ages. Estrogen peaks at age 25 and iron starts to increase from the same age. While estrogen sharply decreases during the menopausal transition period, iron dramatically increases during the same period. DII® may be more age defying in young women but more disrupting with higher success rate in older women. Man starts to accumulate iron from the 20s to the 30s and skin pigmentation is higher in man than in woman (Rahrovan et al. 2018). As a result, DII® is also applicable to man.
References:
Jian J, Pelle E, Yang Q, Pernodet N, Maes D, Huang X. (2011). Iron sensitizes keratinocytes and fibroblasts to uva-mediated matrix metalloproteinase-1 through tnf-alpha and erk activation. Exp Dermatol 20:249-254. https://www.ncbi.nlm.nih.gov/pubmed/20701626
Pelle E, Jian J, Zhang Q, Muizzuddin N, Yang Q, Dai J, et al. (2013). Menopause increases the iron storage protein ferritin in skin. J Cosmet Sci 64:175-179. https://www.ncbi.nlm.nih.gov/pubmed/23752032
Pierre JL, Fontecave M. (1999). Iron and activated oxygen species in biology: The basic chemistry. Biometals 12:195-199. https://www.ncbi.nlm.nih.gov/pubmed/10581682
Pourzand C, Watkin RD, Brown JE, Tyrrell RM. (1999). Ultraviolet a radiation induces immediate release of iron in human primary skin fibroblasts: The role of ferritin. Proc Natl Acad Sci U S A 96:6751-6756. https://www.ncbi.nlm.nih.gov/pubmed/10359784
Rahrovan S, Fanian F, Mehryan P, Humbert P, Firooz A. (2018). Male versus female skin: What dermatologists and cosmeticians should know. Int J Womens Dermatol 4:122-130. https://www.ncbi.nlm.nih.gov/pubmed/30175213
![]()
 About the Author
About the Author
Biography: Xi Huang has investigated iron’s role in diseases for more than three decades and is credited in more than 90 peer-reviewed publications, many of which demonstrate that excess iron is an important risk factor in women’s health. Dr. Huang was a faculty member at New York UniversitySchool of Medicine and his research laboratory has shown that iron accumulation due to the cessation of menstruation in postmenopausal women contributes to osteoporosis and skin aging. Dr. Huang is the founder and president of FE:I Beauty Tech, the parent company of i-On Skincare (www.ionskincare.com). Dr. Huang received his Ph.D. and M.S. in Toxicology and Applied Pharmacology from the Université Denis Diderot – Paris VII and received his undergraduate degree from China Agricultural University.


