Cosmetic chemists are an innovative, curious, and creative group of scientists, continually looking to formulate the most effective and pleasing products for the world’s consumers. Yet when asked to create the galenic forms most often requested by marketing – creams and lotions – the “default emulsion” is almost always oil-in-water (O/W). While O/W systems offer good sensory properties and ease of manufacturing, the primary alternative system – water-in-oil (W/O) – offers distinct advantages, among them long-lasting adherence to the skin and improved water resistance. So why isn’t W/O used more often? Because (a) it is difficult to create a stable W/O system, and (b) the esthetics of a W/O emulsion are often undesirable (sticky, tacky, thick…).
Let’s cover some key concepts to enhance stability when formulating W/O emulsions:
- When making W/O emulsions, high energy is required
Why do we need high energy when making a W/O emulsion? High energy brings several benefits to your W/O system, altogether contributing to a stable emulsion:
- Creates high energy dissipation rates 1,2
- Controls particle size of the dispersed phase of the emulsion 1,2
- Reduces interfacial tension 1,2
To achieve the desired results, one may use high-energy equipment such as a rotor stator/homogenizer or a deflocculator at moderate to high shear.
Let’s take a closer look at each…
A) Dissipation rate refers to the rate of conversion of turbulence into heat by molecular velocity.
Here is a simplified energy flow chart when you are creating a W/O emulsion:
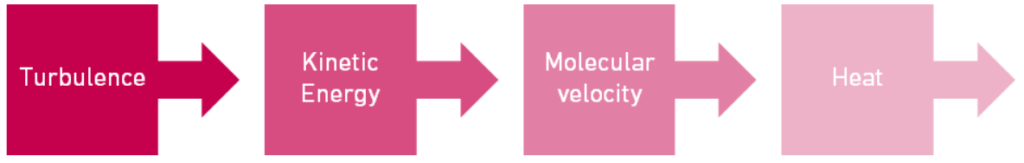
Turbulence, initiated by the high-shearing rotor stator, transfers its energy into kinetic energy within the W/O system. That energy of motion is converted into the large velocity gradients of the dispersed droplets of various sizes. And finally, the energy of the rapidly moving particles is converted into heat through dissipation.2
This is a very simplified flowchart, as there are many other variables involved, but the take-home message here is that the higher the conversion of energy into heat, the higher the dissipation energy, the more kinetically stable emulsion. Essentially, we do not want any energy left in the emulsion, especially in those water droplets because that can lead to instability.
B) The energy applied when creating a W/O emulsion affects the particle size of the internal phase of an emulsion.
As mentioned before, high energy processes will use rotor stators/homogenizers, while a low energy process may use simpler blades (turbine stirrer, propeller, or blade stirrer). If you use high energy, you will achieve a finer, more evenly dispersed emulsion, which is exactly what you want, as this is more stable.
But if homogenization is too mild, due to your equipment and/or shearing speed, your W/O emulsion will be highly poly-dispersed, meaning, there will be a wide size distribution in the dispersed droplets, which can lead to instability.
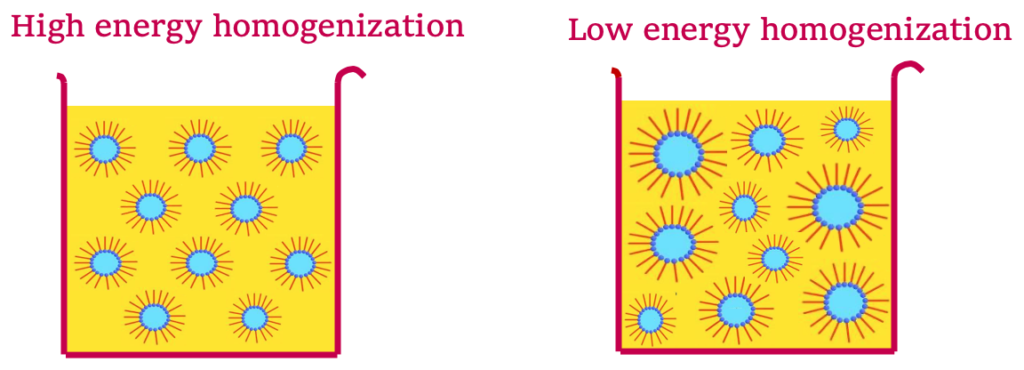
C) And finally, we need to use high energy with W/O systems because it reduces the interfacial tension (energy present at the water-oil interface).
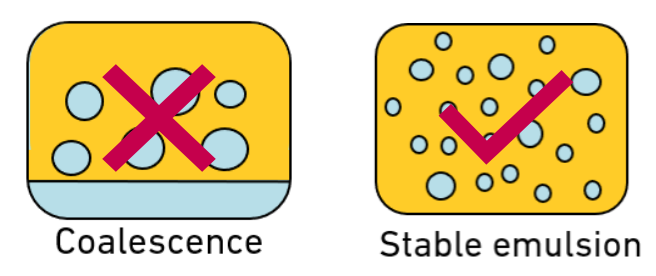
This benefit is a result of the other two: The more heat that is given off, the less energy and mobility in the dispersed water phase. And the smaller the average droplet size of the dispersed phase, the more the stability increases. Altogether, there is less energy remaining at the water-oil interface, so water droplets are less likely to coalesce and will remain stable within the continuous oil phase.
- Electrolytes must be used
Electrolytes – inorganic salts such as magnesium sulfate or sodium chloride – must be present in the emulsion because they will stabilize your system through various mechanisms of action. For example, NaCl has been shown to decrease the particle size through electrostatic and steric repulsion in the droplets.3 CaCl2 has proven to decrease attractive forces between water droplets.3 And MgCl2 can reduce interfacial tension and enhance interfacial film strength.3
Each of these mechanisms may prevent one or more of the following from happening.
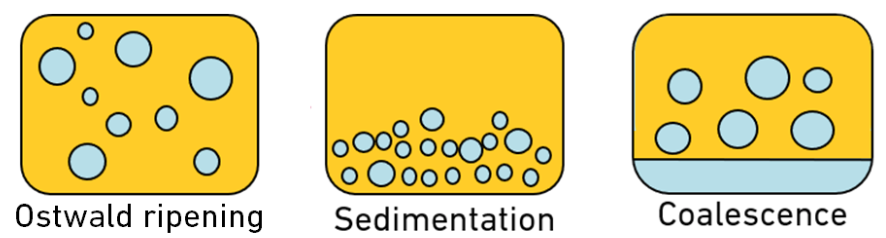
1) Ostwald ripening, also referred to as disproportionation, is caused by the difference in solubility in emulsion droplets. Smaller droplets are more soluble than larger ones, and with prolonged time, the smaller droplets tend to diffuse in the bulk and are deposited on larger droplets. Therefore, larger droplets eventually grow at the expense of smaller ones. Adding salts will counterbalance the driving force for Ostwald ripening, which is related to the total pressure and pressure in the droplets.3
2) Sedimentation is another unstable condition where there is no change to the droplet size, but droplets move to the bottom. The addition of various salts could improve stability by decreasing particle size and reducing the interfacial tension. Salts can allow for tighter packing of surfactant molecules at the O/W interface.3
3) Coalescence is when droplets join, creating larger sized droplets with water separation at the bottom. Adding salts will reduce the attractive forces between water droplets, which will reduce their collision frequency, and thereby prevent droplet coalescence and increase emulsion stability.3
- Depending on the emulsifier, the polarity of the oils used must be specific
Unlike O/W systems, the polarity of the oils used in the oil phase has an outsized influence on the stability of the emulsion, and the performance of high-polarity vs. low-polarity oils will be significant. This is because of the “like dissolves like” rule. In general, chemicals of similar polarities demonstrate better interaction. If the lipophilic tails of your emulsifier are polar, perhaps having esters or hydroxyl groups in its carbon backbone, for example, then the emulsifier is better suited in an oil phase of medium to high polarity. The opposite holds true as well. If the polarity of the oil phase does not match that of the emulsifier, the emulsion will not be stable.
Now, let’s talk briefly about the esthetics of W/O emulsions
W/O emulsions are often tacky or draggy, leaving an unpleasant skin feel and an uncomfortably thick layer of product. Also, as W/O systems are often used with pigments, many common emulsifiers do not have the correct compatibility with the wide variety of pigments used today, resulting in non-homogenous dispersions of the pigments within the emulsion. Many traditional W/O emulsifiers were not designed to address issues of skin feel or pigment dispersion, but modern advances in esterification chemistry allow for the creation of a new generation of emulsifiers that provide perceptibly improved sensory characteristics.
It can be suggested that the use of an emulsifier based on polyglycerol chemistry is especially suited to W/O systems due to enhanced stability resulting from large polar headgroups. (Incidentally, polyglycerol chemistry is considered “green” and advantageous when formulating natural or clean products…) Esterifying a polyglycerol backbone with other esters will significantly effect both skin feel and pigment dispersion properties; for example, the use of a ricinoleic acid ester could provide fluidity and improved skin feel, while the use of a hydroxystearic acid ester could improve the dispersibility of both coated and uncoated pigments.
With these concepts in mind, formulating a W/O emulsion can result in an elegant product satisfying the end consumer while meeting the requirements of marketing, allowing the creativity of the chemist to move “beyond the box” of traditional cosmetic emulsions.
References
- Turbulence and multiphase flow. http://www.lowshearschool.com/?page_id=16919
- The effect of shear on oil-water mixture. http://www.lowshearschool.com/?page_id=16933
- Zhu Q , Pan Y, Jia X, Li J, Zhang M, Yin L. Review on the stability mechanism and application of water-in-oil emulsions encapsulating various additives. Comprehensive Reviews in Food Science and Food Safety, 18 (6): 1660-1675, 2019
 Leor Fay Tal is the Technical Marketing Leader for the Personal Care division of Gattefossé USA. She delivers information on trends and consumers, provides technical marketing support to the company’s sales teams and agents across North America, Canada, and Mexico, and works to promote knowledge and understanding of the company’s ingredients. Prior to Gattefossé, Leor Fay had worked in the R&D Powder Laboratory and then as the Raw Material Regulatory Affairs Specialist at MANA Products. Leor Fay is also an active member of the NYSCC. She organized the April 2018 event Cosmetics in the Middle East, A Regulatory Perspective and now serves as the Secretary for the executive board.
Leor Fay Tal is the Technical Marketing Leader for the Personal Care division of Gattefossé USA. She delivers information on trends and consumers, provides technical marketing support to the company’s sales teams and agents across North America, Canada, and Mexico, and works to promote knowledge and understanding of the company’s ingredients. Prior to Gattefossé, Leor Fay had worked in the R&D Powder Laboratory and then as the Raw Material Regulatory Affairs Specialist at MANA Products. Leor Fay is also an active member of the NYSCC. She organized the April 2018 event Cosmetics in the Middle East, A Regulatory Perspective and now serves as the Secretary for the executive board.
 Ben Blinder is the Senior Director for Gattefossé USA – Personal Care Division, where he is responsible for the strategic direction and performance of the cosmetic business for Gattefossé in the US and Mexico. Ben holds a chemical engineering degree from Lehigh University and has been working in the personal care industry for 32 years, with extensive experience in strategic and long-range planning, sales and technical management, and new technology search/discovery. Ben also serves on the NYSCC Scientific Committee.
Ben Blinder is the Senior Director for Gattefossé USA – Personal Care Division, where he is responsible for the strategic direction and performance of the cosmetic business for Gattefossé in the US and Mexico. Ben holds a chemical engineering degree from Lehigh University and has been working in the personal care industry for 32 years, with extensive experience in strategic and long-range planning, sales and technical management, and new technology search/discovery. Ben also serves on the NYSCC Scientific Committee.
